What Single-Cell Transcriptomics Is Revealing About Alzheimer’s Disease Cells
 Nuhash Gazi
•
Nuhash Gazi
•
Introduction
As single-cell transcriptomics has been applied to Alzheimer’s disease, it has started to transform our understanding of how different brain cells respond to pathology. Rather than seeing the brain as a uniform tissue affected in the same way, we now see a complex landscape of vulnerable and resilient cell populations.
Recent Advances in Single-Cell Transcriptomics for Alzheimer’s Disease
Recent studies have applied single-cell transcriptomics to post-mortem brain samples from Alzheimer’s patients and healthy controls. These efforts have uncovered neuron subtypes that are particularly vulnerable to degeneration, offering clues about why memory-related regions deteriorate first. Some neuron populations show early gene expression changes long before extensive cell death, indicating that they enter a stressed state that may be reversible if targeted in time.
Microglia, the immune cells of the brain, have also been shown to adopt specialized states in Alzheimer’s disease. Some of these states promote inflammation and may worsen damage, while others attempt to clear harmful proteins such as amyloid-beta. Single-cell data allow researchers to define these states precisely and link them to specific genes and pathways.
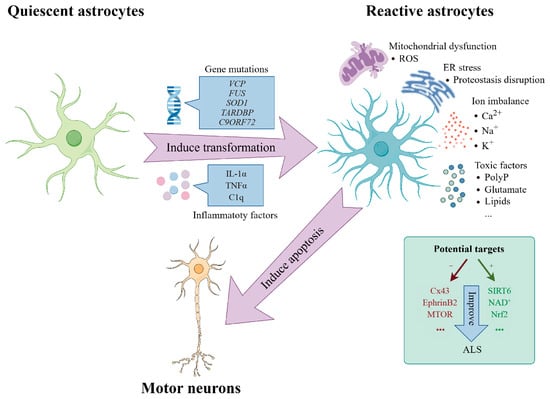
Astrocytes have emerged as another key player. Traditionally seen as support cells, astrocytes are now recognized as active regulators of brain health. Single-cell studies have identified astrocytic programs that appear to support resilience against cognitive decline, suggesting that targeting them might help preserve function even when pathology is present.
Multi-region brain atlases created with single-cell techniques have further highlighted how gene expression changes differ across areas of the brain. Some regions show early immune activation, while others show synaptic loss or metabolic stress. These atlases provide a detailed map of how Alzheimer’s pathology spreads and evolves across the brain.
Toward Non-Invasive Biomarkers
A growing area of research links single-cell signatures in the brain to cell-free RNA found in blood or cerebrospinal fluid. If specific cellular states in the brain leave a distinct “fingerprint” in circulating RNA, these could serve as non-invasive biomarkers.
In my opinion, this represents one of the most exciting directions, as it bridges basic research with clinical application. Instead of waiting for severe symptoms or relying only on imaging, clinicians might one day monitor cellular changes indirectly through a blood or fluid test that reflects what is happening inside the brain.
Conclusion
Overall, recent advances show that Alzheimer’s disease is not a uniform process but a highly cell-type-specific and region-specific condition. Single-cell transcriptomics is revealing which cells change first, which become harmful, and which attempt to protect the brain. These insights provide a crucial foundation for designing more targeted and effective therapies, which I will explore in the next part of this series.
As single-cell transcriptomics has been applied to Alzheimer’s disease, it has started to transform our understanding of how different brain cells respond to pathology. Rather than seeing the brain as a uniform tissue affected in the same way, we now see a complex landscape of vulnerable and resilient cell populations.
Recent Advances in Single-Cell Transcriptomics for Alzheimer’s Disease
Recent studies have applied single-cell transcriptomics to post-mortem brain samples from Alzheimer’s patients and healthy controls. These efforts have uncovered neuron subtypes that are particularly vulnerable to degeneration, offering clues about why memory-related regions deteriorate first. Some neuron populations show early gene expression changes long before extensive cell death, indicating that they enter a stressed state that may be reversible if targeted in time.
Microglia, the immune cells of the brain, have also been shown to adopt specialized states in Alzheimer’s disease. Some of these states promote inflammation and may worsen damage, while others attempt to clear harmful proteins such as amyloid-beta. Single-cell data allow researchers to define these states precisely and link them to specific genes and pathways.
Astrocytes have emerged as another key player. Traditionally seen as support cells, astrocytes are now recognized as active regulators of brain health. Single-cell studies have identified astrocytic programs that appear to support resilience against cognitive decline, suggesting that targeting them might help preserve function even when pathology is present.
Multi-region brain atlases created with single-cell techniques have further highlighted how gene expression changes differ across areas of the brain. Some regions show early immune activation, while others show synaptic loss or metabolic stress. These atlases provide a detailed map of how Alzheimer’s pathology spreads and evolves across the brain.
Toward Non-Invasive Biomarkers
A growing area of research links single-cell signatures in the brain to cell-free RNA found in blood or cerebrospinal fluid. If specific cellular states in the brain leave a distinct “fingerprint” in circulating RNA, these could serve as non-invasive biomarkers.
In my opinion, this represents one of the most exciting directions, as it bridges basic research with clinical application. Instead of waiting for severe symptoms or relying only on imaging, clinicians might one day monitor cellular changes indirectly through a blood or fluid test that reflects what is happening inside the brain.
Conclusion
Overall, recent advances show that Alzheimer’s disease is not a uniform process but a highly cell-type-specific and region-specific condition. Single-cell transcriptomics is revealing which cells change first, which become harmful, and which attempt to protect the brain. These insights provide a crucial foundation for designing more targeted and effective therapies, which I will explore in the next part of this series.